Abstract
Background: Myelodysplastic syndrome (MDS) is a group of disorders of the hematopoietic stem cells characterized by ineffective hematopoiesis, cytopenias, genetic abnormalities, and an increased risk of progression to acute myeloid leukemia (AML). In addition, about half of patients with long lasting cytopenia have clonal hematopoiesis with mutations in genes that are also mutated in myeloid malignancies (i.e. clonal cytopenia of undetermined significance; CCUS) and many develop MDS and AML within 1-10 years. Myeloid-derived suppressor cells (MDSC) are immune suppressing cells that infiltrate the bone marrow (BM) microenvironment in MDS and an increased number of MDSC is associated with disease progression.
The presence of self-reactive effector T cells, termed anti-regulatory T cells (anti-Tregs), has been evidenced in cancer patients. These anti-Tregs can target immunosuppressive cells, such as MDSC, by recognizing epitopes derived from immunoregulatory proteins including indoleamine 2,3-dioxygenase (IDO), arginase-1 (ARG) and TGFβ. Thus, we hypothesize that the activation of anti-Treg using peptide-based vaccines can lead to the depletion of the immunosuppressive cells in the BM, and it can help to prevent disease progression in patients with MDS or CCUS.
Aim: To study the efficacy of anti-Treg stimulation as an immunotherapy in patients with MDS or CCUS.
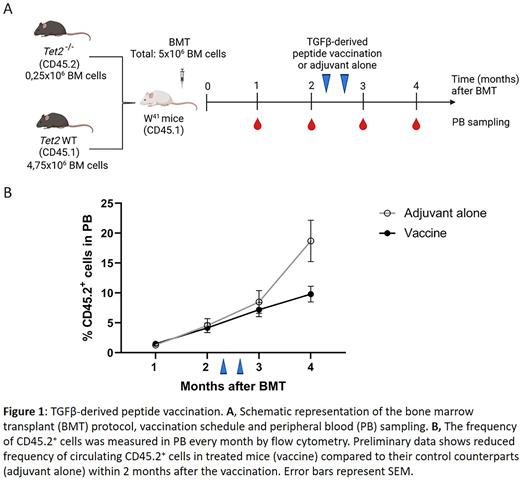
Methods: We performed extensive flow cytometry analysis of BM and peripheral blood (PB) samples from 20 patients (pts) (10 CCUS and 10 low-risk MDS pts) and 6 aged-matched healthy controls (HC). The presence of anti-Tregs was investigated in 7 pts, by ELISPOT assay on pts' T cells that had been stimulated with epitopes derived from ARG, IDO or TGFβ. The Tet2fl/fl;R26-CreER mouse strain was used to obtain a cohort of Cre+Tet2Lox/Lox (hereafter Tet2−/−) and control Cre-Tet2Lox/Lox (hereafter Tet2+/+) animals that were injected with Tamoxifen to inactivate Tet2 in adult mice. We transplanted Tet2+/+ or Tet2−/− (CD45.2) BM cells into unconditioned W41 recipients (CD45.1) and followed the engraftment/expansion of transplanted cells in PB by measuring the frequency of CD45.2+ cells by flow cytometry. For therapeutic experiments, W41 mice transplanted with Tet2−/− cells were randomly assigned and treated with a TGFβ-derived peptide vaccine, containing epitopes recognized by both CD4 and CD8 T cells mixed with adjuvant (Montanide), or adjuvant alone (2 doses, subcutaneously, 10 days apart). Complete blood counts and frequency of CD45.2+ cells were measured every month in PB.
Results: The analysis of BM samples showed that the proportion of IDO+ and ARG+ MDSC in pts was significantly higher compared to HC. In the ELISPOT assay, 4 out the 7 pts showed a significant response towards the ARG peptide, 2 showed a response towards IDO peptide and in 4 of the patients there was a significant response towards the TGFβ peptide. Overall, of the 7 pts analyzed, six had T cells specific to one or more of the immunosuppressive proteins tested, and the highest response observed was against the TGFβ peptide. To develop a mouse model of CCUS/low-risk MDS we generated a cohort of mice with a hematopoietic system with low variant allele frequency of Tet2−/− clones by transplanting 0,25x106Tet2+/+ or Tet2−/− BM cells together with 4,75x106 competitor BM cells into unconditioned W41 mice (Figure 1A). We observed an engraftment of Tet2−/−, but not Tet2+/+ cells, and an expansion of the clone over time. Within two months after the BM transplant, the mean percentage of Tet2−/− cells in the blood was 3,4%, mimicking clonal hematopoiesis in patients. W41 animals that received the Tet2-/- BM transplant were treated with a TGFβ-derived peptide vaccine to induce the activation of anti-Treg cells. Preliminary data shows reduced frequency of circulating CD45.2+ cells in the treated mice compared to their control counterparts within 2 months after the vaccination (Figure 1B).
Conclusion: There is an increased proportion of activated MDSC in BM in pts with CCUS/MDS compared to HC. The ELISPOTs showed that circulating anti-Tregs are present in these pts. We generated a mouse model that recapitulates Tet2-driven clonal hematopoiesis, and our preliminary results suggest that the activation of TGFβ-specific T cells by an immune-modulatory vaccination could be a promising strategy to prevent the expansion of mutated HSC clones in the context of clonal hematopoiesis and CCUS/MDS.
Disclosures
Andersen:IO Biotech: Consultancy, Current equity holder in publicly-traded company. Grønbæk:Janssen: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Nanexa: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal